COVID-19 is a worldwide pandemic. To solve this crisis, scientists, pharmaceutical companies, and biotech companies—such as AstraZeneca, Moderna, Pfizer, BioNTech, Novavax and J&J—are currently working on finding cures. The world’s urgent need for a vaccine has never been seen before. However, many vaccine developing companies might not notice that they are walking straight into a future patent war.
This article explains why there will be a patent war in the near future and provides advice for those vaccine developing companies that are participating in or are going to participate in the race for vaccine development.
The Race for COVID-19 Vaccine Development
The coronavirus pandemic is having a significant impact on the whole world and humanity. There have been 20.2 million confirmed cases since December 2019, including over 730,000 deaths (as of August 13th, 2020). Although seven treatments have been approved for emergency use—such as Remdesivir—these treatments can only reduce the number of fatal cases, they can not eradicate the virus. The best chance of putting a stop to this crisis is to find an effective vaccine.
According to the COVID-19 Therapeutic Development Tracker—made by the Biotechnology Innovation Organization (BIO)—many pharmaceutical companies and biotech companies across the globe are participating in the race to develop a vaccine to end this pandemic.
There are currently 181 vaccines in development, out of which 34 are in different stages of clinical trials— this number continues to increase every week. The five candidates that are attracting the most attention include:
- AZD-1222 is one of the leading COVID-19 vaccine candidates developed by AstraZeneca (NYSE: AZN) and the University of Oxford. It comprises a recombinant adenovirus vector expressing the spike protein of SARS-CoV-2 and is currently in its large-scale phase 3 clinical studies.
- mRNA-1273 is an mRNA-based vaccine developed by a US company called Moderna Therapeutics (NASDAQ: MRNA). The company just started its large-scale phase 3 clinical studies in mid-July. According to the company’s prediction, a positive result could happen as soon as November.
- BNT162 is an mRNA vaccine developed by Pfizer (NYSE: PFE) and BioNTech SE (NASDAQ: BNTX). BNT162 entered its phase 1 trial as multiple related candidates. One of the candidates—BNT162b2—was chosen to commence a phase 2b/3 trial in late-July.
- NVX-CoV2373 is a protein-based vaccine comprising the spike protein of SARS-CoV-2 with a patented adjuvant technology called Matrix-M—developed by a US biotech company, Novavax. The company is working on a phase 1/2 clinical trial and expects to begin a phase 3 study this fall.
- Ad26.COV2.S is an adenovirus-based vaccine developed by Johnson and Johnson (NYSE: JNJ). This vaccine candidate expresses SARS-CoV-2 spike protein in the human body—using Janssen’s AdVac and PER.C6 technologies—which the company has used in other vaccines. The company started its phase 1/2a clinical trials with the candidate in late-July.
If everything goes well, some of the above-mentioned vaccine candidates may be available for emergency use in early-2021. However, even though the data from the pre-clinical/clinical studies look promising so far, it is still hard to tell whether the protection rates of these vaccines will be good enough to end the pandemic before these vaccines are used on the public (according to the FDA, the requirement for the COVID-19 vaccine protection rate should not be less than 50%). Additionally, those leading companies’ manufacturing capacity might not be sufficient to provide enough vaccines for the whole world. So even though the pipeline is already crowded, new companies are still jumping into the race for COVID-19 vaccine development.
The Unseeable Patent Risk of Developing a COVID-19 Vaccine
Checking existing patents to avoid potential infringement risk before initiating product development is almost a standard procedure for pharmaceutical companies. However, the pandemic has made the situation very different.
In major countries around the world, patent applications are usually published 18 months after patents are filed. The pandemic started at the end of 2019; therefore, most patent applications that were filed during the pandemic are still not published yet. This situation causes the COVID-19 treatment developers to develop their own products without knowing about patents that others may have filed in this period. We can foresee that there will be many COVID-19 related patent applications coming to light in mid-2021.
When companies are devoted to developing COVID-19 vaccine candidates, they might not be aware that they are also walking into a potential future patent war. To control the risk of future patent infringement for the vaccine developers, we have the following advice.
1 — Monitor
Continuously monitor the patent risk for the developing candidates during the pandemic; assessing the risk of potential infringement should not be a one-time job. For those patents that have not yet been published, developers should constantly monitor whether new patents related to their products have been published. To make this task easier, we should conduct a patent search and keep the results up-to-date with the help of a patent search engine capable of auto-monitoring, such as Patent Search by Patentcloud.
When should we start the search?
Even though we can’t find the patent applications filed during the pandemic yet, it is still necessary to conduct a patent search for the developing candidates at this time. The reason is that there could be some existing patents related to the general technologies for the vaccines, for example, technologies for vaccine delivery, vaccine production, vector system, drug formulation, and adjuvant—even vaccines for other coronaviruses, such as SARS or MERS, which may be a potential risk for the developing candidates.
2 — Invalidate
Seek patent invalidation if there is a potential infringement risk. If a patent that has the potential of being infringed upon by the developing candidate is found, it would be a problem to perform a design around if the candidate is already in the clinical stage.
Since testing drugs on humans is highly regulated, changing the design of a vaccine might lead to all previous and ongoing clinical trials restarting from zero. Therefore, a design around may not be an option in this situation.
To deal with potential infringement risks, patent invalidation could be a solution. One of the most recent examples is that Moderna attempted to invalidate a patent related to the lipid nanoparticle (LNP) technology owned by Arbutus Biopharma. The vaccine developer filed an inter partes review against U.S. Patent No. 8,058,069 (‘069 patent) patented by Arbutus Biopharma. Unfortunately, the U.S. Patent Trial and Appeal Board (PTAB) ruled that Moderna had not established by a preponderance of the evidence that the claims of the disputed patent were anticipated or rendered obvious (IPR 2019-00554).
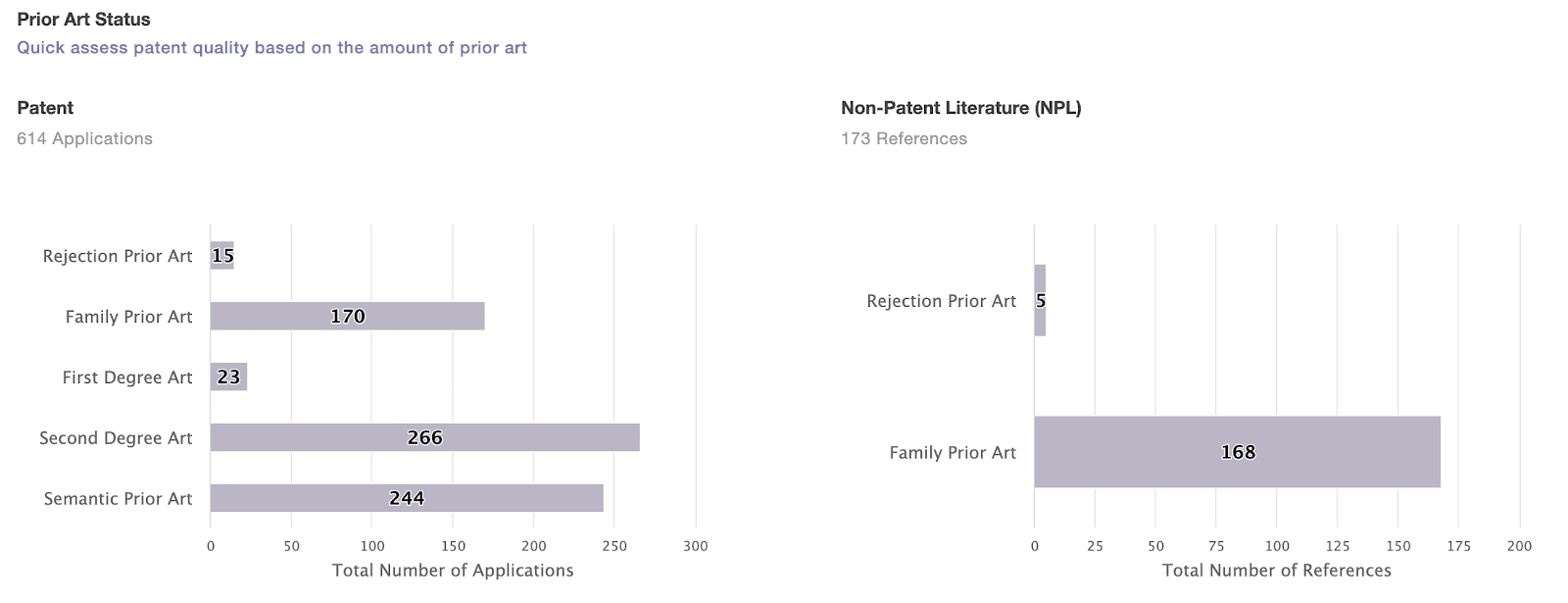
Moderna can still appeal the decision to the United States Court of Appeals for the Federal Circuit (CAFC) or file another post-granted review with different prior art and grounds to invalidate the ‘069 patent. To quickly collect potential prior art for patent invalidation, there are several aspects that we can look into:
The prior art used by examiners to reject the counterparts in the same patent family of the disputed patent—especially if the counterparts were abandoned or revoked.
The secondary citations of the prior art used in the prosecution history or litigations.
The prior art collected based on the semantic similarity of the abstracts and claims to the first claim of the patent at issue, using the world’s first one-click solution for patent validity analysis, Quality Insights by Patentcloud.
3 — Develop
As we can see from the current COVID-19 vaccine candidates, many companies share similar technology solutions in their developing vaccine products. In this case, the situation could be more desirable if one owns patents that could be infringed by one’s competitor, especially when it is impossible to avoid potential infringement by patent invalidation. These patents could then be utilized as bargaining chips for negotiating cross-licensing.
If you are a vaccine development company, discover more about how to mitigate the potential risk of being involved in a future patent war now by taking advantage of a free trial of Quality Insights!